Tuberculosis
Introduction
Tuberculosis (TB) is a bacterial infection caused by Mycobacterium tuberculosis and is transmitted primarily by inhalation.
- After primary infection, 90% of individuals with intact immunity control further replication control of the bacilli, which may then be cleared or enter a "latent" phase. The person remains asymptomatic, but latent disease has the potential to become active at any time.
- The remaining 10% of individuals develop progressive to primary tuberculosis. It most commonly affects the lungs, although it can affect other parts of the body.
NOTE: In general, individuals with pulmonary and laryngeal TB are infectious (higher risk if sputum smear is positive), whereas those with extrapulmonary TB are regarded as non-infectious.
Clinical Presentation
Pulmonary TB is characterized by its slow onset and initial mild symptoms.
- The cough is chronic in nature and sputum production can vary from mild to severe with associated haemoptysis.
- Other symptoms of the condition are malaise, fever, night sweats and weight loss.
- However, not all patients will experience all symptoms.
On the other hand, symptoms and signs due to extrapulmonary TB vary according to the organs involved and may be non-specific.
Diagnostic Tools
- Chest X-ray
- Sputum acid-fast bacilli (AFB) smear
- Nuclear acid amplification testing (NAA)
- Tuberculin skin test (Mantoux test or TST)
- Interferon-γ release assay (IGRA)
Management for Drug-Susceptible Tuberculosis
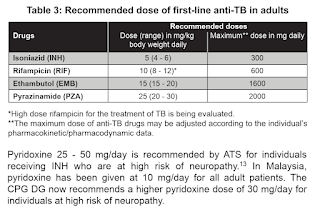
Presently, six-month regimen consisting of 2 months of daily EHRZ (intensive phase), followed by 4 months of daily HR (maintenance phase) is recommended for newly diagnosed pulmonary TB.
- Rifampicin and isoniazid should be taken with an empty stomach to have better absorption.
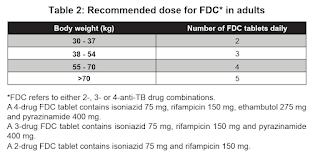
WHO recommends the use of fixed-dose combination (e.g. Akurit-4 and Akurit-2) over separate drug formulations in the treatment of patients with drug-susceptible tuberculosis.
NOTE: Multiples of 18
NOTE: The currently available adult anti-TB fixed dose combination tablet is not suitable for use in children <25 kg.
NOTE: Based on WHO operational handbook on tuberculosis, 2022, the upper limit of ethambutol in adult is 25 mg/kg, but daily maximum dose remains 1600 mg.
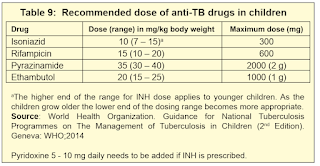
NOTE: Medication dose requires recalculation every 2 to 4 weeks as children gain weight rapidly, particularly in neonates and young children.
On the other hand, all extrapulmonary TB should be treated with anti-TB for a minimum of 6 months except for bone (including spine) and joint tuberculosis for 6-9 months and tuberculosis for 9-12 months.
Renal Impairment
The clearance of ethambutol and pyrazinamide metabolites are impaired in patients with chronic renal failure when their creatinine clearance falls below 30 ml/min.
- Hence, thrice weekly dosing is recommended in international guidelines.
Conversely, rifampicin and isoniazid do not require dose adjustment in renal failure as they are metabolized by the liver.
Drug Counselling
Rifapentine-Moxifloxacin Four-Month Regimen
While traditional regimen remains standard of care, the shortened 4-month rifapentine-moxifloxacin regimen (intensive phase of 8 weeks and continuation phase of 9 weeks) may be used in a subset of patients who fulfill specific criteria:
- Age ≥12 years
- Body weight ≥40 kg
- Drug-susceptible pulmonary tuberculosis, in the absence of extrapulmonary involvement
The 4-month regimen should not be used for patients in the following categories:
- Patients with suspected or confirmed resistance to the medications in the regimen.
- Patients with history of cardiac morbidities (given risk for fluoroquinolone-induced toxicity)
- Patients with advanced liver disease or renal insufficiency
- Pregnant or lactating women
Drug-Resistant Tuberculosis
Definitions for drug-resistant tuberculosis
- Rifampin-resistant TB - An isolate of M. tuberculosis with resistance to rifampin.
- Multidrug-resistant TB (MDR-TB) - An isolate of M. tuberculosis with resistance to at least isoniazid and rifampin and possibly additional antituberculous agents.
- Pre-extensively drug-resistant TB (pre-XDR-TB) - An isolate of M. tuberculosis with resistance to isoniazid and rifampin as well as fluoroquinolone (levofloxacin or moxifloxacin)
- Extensively drug-resistant TB (XDR-TB) - An isolate of M. tuberculosis with resistance to isoniazid, rifampin, a fluoroquinolone (levofloxacin or moxifloxacin) and either bedaquiline or linezolid.
WHO suggests the use of a 6-month treatment regimen composed of bedaquiline, pretomanid, linezolid (600 mg) and moxifloxacin (BPaLM) rather than the 9-month or longer (18-month) regimens in MDR/RR-TB patients.
Remarks:
- Drug susceptibility testing (DST) for fluoroquinolones is strongly encouraged in people with MDR/RR-TB, and although it should not delay initiation of the BPaLM, results of the test should guide the decision on whether moxifloxacin can be retained or should be dropped from the regimen - in cases of documented resistance to fluoroquinolones, BPaL without moxifloxacin would be initiated or continued.
- This recommendation applies to the following:
- People with MDR/RR-TB or with MDR/RR-TB and resistance to fluoroquinolones (pre-XDR-TB).
- People with confirmed pulmonary TB and all forms of extrapulmonary TB except for TB involving the CNS, osteoarticular or disseminated forms of TB with multiorgan involvement.
- Adults and adolescents aged 14 years and older.
- All people regardless of HIV status.
- Patients with less than 1-month previous exposure to bedaquiline, linezolid, pretomanid or delamanid. When exposure is greater than 1 month, these patients may still receive these regimens if resistance to the specific medicines with such exposure has been ruled out.
- This recommendation does not apply to pregnant and breastfeeding women owing to limited evidence on the safety of pretomanid.
WHO suggests the use of the 9-month all-oral regimen rather than longer (18-month) regimens in patients with MDR/RR-TB and in whom resistance to fluoroquinolones has been excluded.
- The 9-month all-oral regimen consists of bedaquiline (used for 6 months), in combination with levofloxacin/moxifloxacin, ethionamide, ethambutol, isoniazid (high-dose), pyrazinamide and clofazimine (for 4 months, with the possibility of extending to 6 months if the patient remains sputum smear positive at the end of 4 months), followed by treatment with levofloxacin/moxifloxacin, clofazimine, ethambutol and pyrazinamide (for 5 months). Ethionamide can be replaced by 2 months of linezolid (600 mg daily).
- A 9-month regimen with linezolid instead of ethionamide may be used in pregnant women, unlike the regimen with ethionamide.
- This recommendation applies to:
- people with MDR/RR-TB and without resistance to fluoroquinolones;
- patients without extensive TB disease32 and without severe extrapulmonary TB;
- patients with less than 1 month exposure to bedaquiline, fluoroquinolones, ethionamide, linezolid and clofazimine; when exposure is greater than 1 month, these patients may still receive this regimen if resistance to the specific medicines with such exposure has been ruled out;
- all people regardless of HIV status;
- children (and patients in other age groups) who do not have bacteriological confirmation of TB or resistance patterns but who do have a high likelihood of MDR/RR-TB (based on clinical signs and symptoms of TB, in combination with a history of contact with a patient with confirmed MDR/RR-TB).
A longer regimen should be used in patients who are not eligible or had no favourable outcome using previous 6- or 9-month regimens.
- In multidrug- or rifampicin-resistant tuberculosis (MDR/RR-TB) patients on longer regimens, all three Group A agents and at least one Group B agent should be included to ensure that treatment starts with at least four TB agents likely to be effective, and that at least three agents are included for the rest of the treatment if bedaquiline is stopped.
- If only one or two Group A agents are used, both Group B agents are to be included.
- If the regimen cannot be composed with agents from Groups A and B alone, Group C agents are added to complete it
Grouping of medicines recommended for use in longer MDR-TB regimens:
- Group A = levofloxacin or moxifloxacin, bedaquiline and linezolid;
- Group B = clofazimine, and cycloserine or terizidone; and
- Group C = ethambutol, delamanid, pyrazinamide, imipenem-cilastatin or meropenem, amikacin (or streptomycin), ethionamide or prothionamide, and p-aminosalicylic acid.
External Links
- How long are TB patients infectious?, 2000
- CPG Management of Tuberculosis, 2021
- CPG Management of Drug Resistant Tuberculosis, 2016
- National Antimicrobial Guideline, 2024
- Paediatric Protocols for Malaysian Hospitals, 2019
- World Health Organization - Tuberculosis
- WHO TB Knowledge Sharing Platform
- WHO consolidated guidelines on tuberculosis: module 4: treatment and care, 2025
- CDC - Tuberculosis
- Four-Month Rifapentine Regimens with or without Moxifloxacin for Tuberculosis, 2021





Comments
Post a Comment