Tablet Excipients
Introduction
Whenever customers/patients were introduced with generic drugs, their first concern will be "are they exactly the same as the innovator brand product?".
- Technically, both innovator brand and generic brand products contain the same quantity of active pharmaceutical ingredients (the substance that we believe to give the therapeutic benefit), but may contain different pharmaceutical excipients, especially preservatives and colourants.
Functions
Pharmaceutical excipients are basically everything other than the active pharmaceutical ingredient. Depending on the intended main function, the excipients used in tablets are subcategorized into different groups. However, many substances used in tablet formulation can be multifunctional.
Antiadherent
- Antiadherents prevent sticking to punches and die walls.
- Binders or adhesives are the substances that promote cohesiveness. It is added to a drug-filler mixture to ensure that granules and tablets can be formed with the required mechanical strength.
Buffer
- Buffers are added to maintain a required pH since a change in pH may cause significant alteration in stability.
Colourant
- Colourants are added to tablets to aid identification and increase stability of light-sensitive drugs.
- Often useful to match colour with flavour to improve the attractiveness of the product.
Coating
- Tablet coatings protect tablet ingredients from deterioration by moisture in the air and make large or unpleasant-tasting tablets easier to swallow.
Disintegrants
- A disintegrant is included in the formulation to ensure that the tablet, when in contact with a liquid, breaks up into small fragments (increase surface area), which promotes rapid drug dissolution.
Filler (or diluent)
- To form tablets of a size suitable for handling, a lower limit in terms of powder volume and weight is required. Therefore, a low dose of a potent drug requires the incorporation of filler into the formulation to increase the bulk volume of the powder and hence the size of the tablet.
Flavour
- Flavouring agents are incorporated into a formulation to give the tablet a more pleasant taste or to mask an unpleasant one.
Glidants
- Glidants are added to the formulation to improve the flow properties of the material which is to be fed into the die cavity and aid in particle rearrangement within the die during the early stages of compression.
Lubricant
- The function of the lubricant is to ensure that tablet formation and ejection can occur with low friction between the solid and the die wall.
Preservative
- To control the microbial bioburden of the formulation.
Sorbent
- Sorbents are substances that are capable of sorbing some quantities of fluids in an apparently dry state. Thus, oils or oil-drug solutions can be incorporated into a powder mixture which is granulated and compacted into tablets.
Sweetener
- Especially used in the chewable, dispersible, sublingual tablet
Important Things
Ideally, pharmaceutical excipients should be inert and safe (no acute or chronic toxicity).
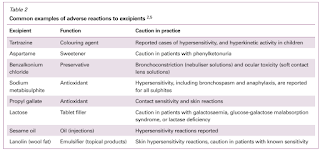
However, in reality, they can cause adverse and hypersensitivity reactions in patients.
Plus, studies have shown that some excipients can have a significant effect on the performance of an oral solid dosage form, including bioavailability and stability.
Also, patients may ask to avoid excipients of animal origin for cultural, religious or lifestyle reasons.
- Some vegans request medicines that exclude substances of animal origin, such as gelatin.
- Jehovah’s Witnesses believe that the Bible prohibits the consumption, storage and transfusion of blood and its associated products.
- Some minor components of plasma (such as, albumin, cryoprecipitate or immunoglobulins) and various clotting factors are not completely forbidden and may be acceptable to some individuals.
- In Hinduism, cattle are considered sacred and it is considered a sin to kill them.
- Therefore, the use of bovine-derived drugs may not be suitable to patients belonging to Hindu communities.
- Porcine (pork) derived products are usually avoided by practising Muslims.
- Muslim patients may also wish to avoid other constituents derived from animals that may not be slaughtered in accordance with Shariah Law.
- Nonetheless, non-halal medicines may be acceptable, if no alternatives exist.
External Links
- Medicines Learning Portal - Excipients
- Pharmaceutical excipients – where do we begin?, 2011
- Excipients Used In the Manufacture of Tablets, 2020
- Implications of religious and cultural beliefs on selection of medicines, 2016
- Avoiding animal contents within medicines, 2025
- Choosing vitamin D products for vegetarians or vegans, 2025
- Panduan Penggunaan Ubat-ubatan yang Mengandungi Unsur Tidak Halal, 2018
- The Vegan Society
- European Vegetarian Union

Comments
Post a Comment